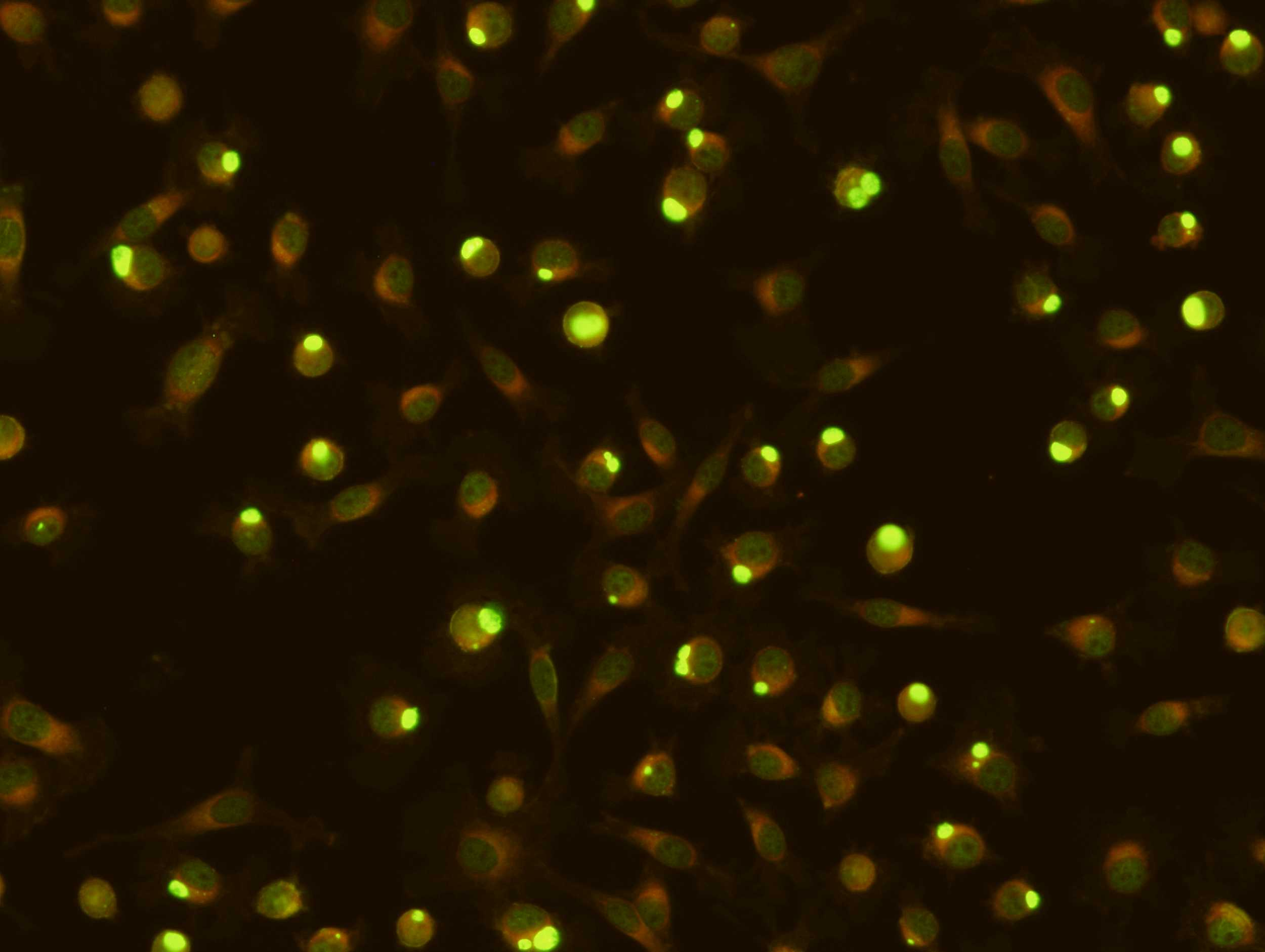
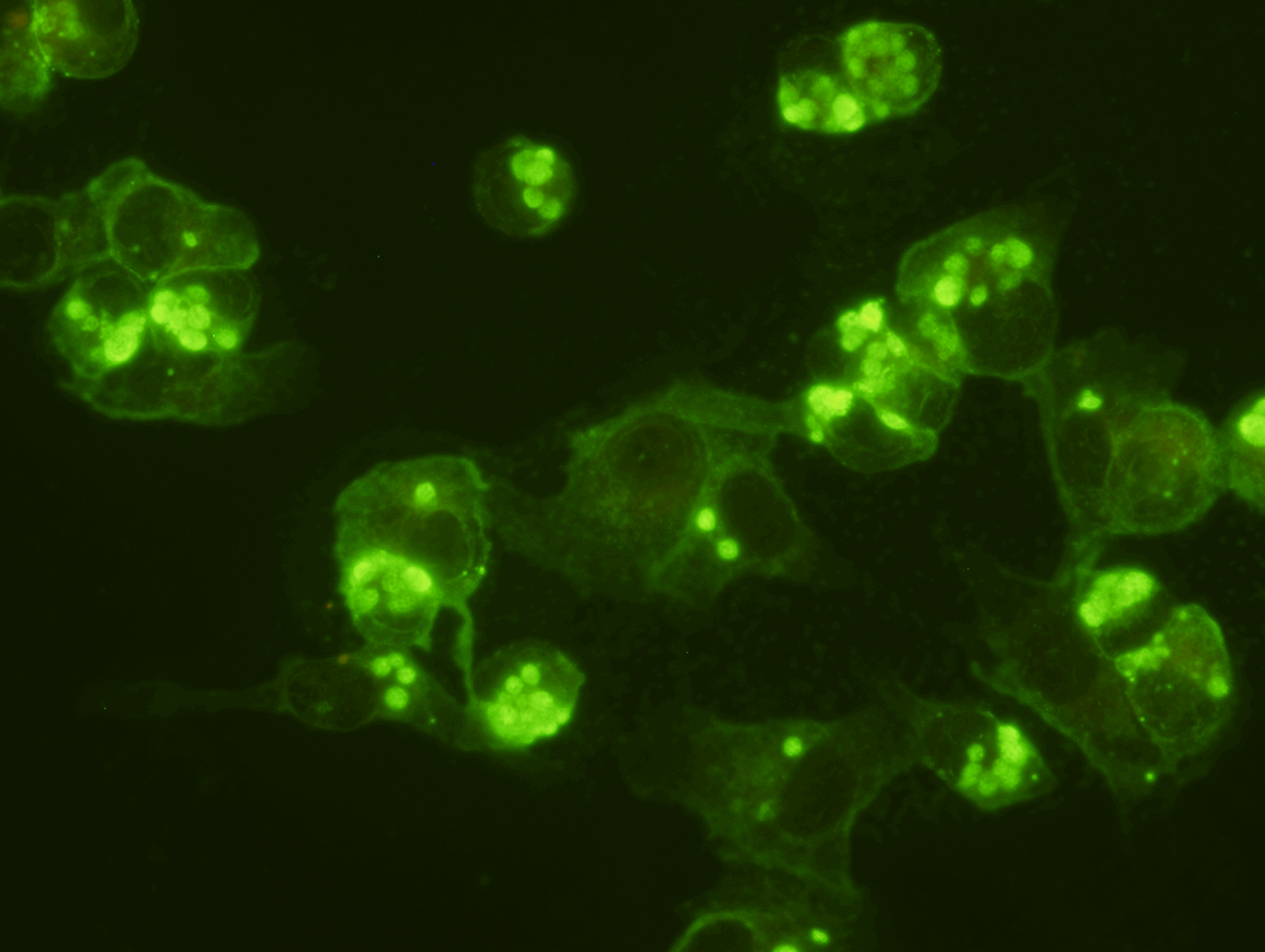
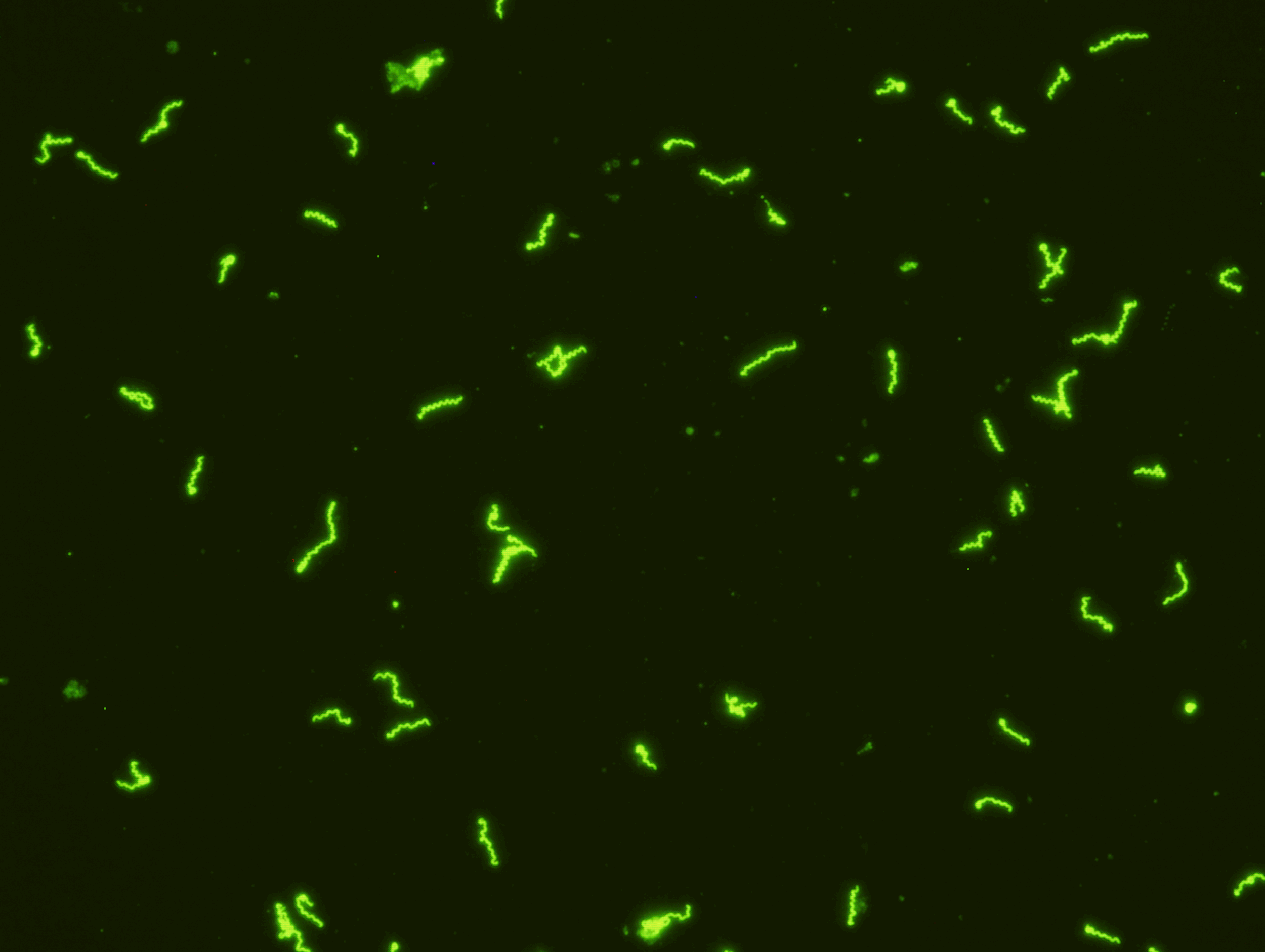
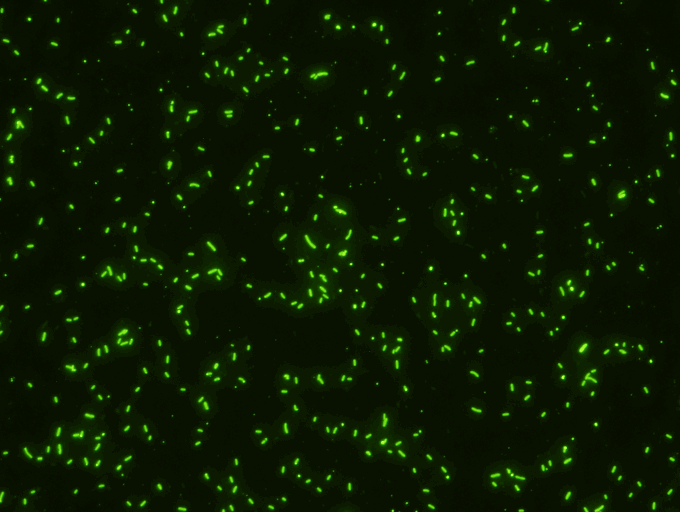
SCIMEDX CORPORATION is a highly flexible diagnostic company, with over 35 years experience in the area of In Vitro Diagnostics. The strength of the organization is the collective diversified experiences of the personnel in many areas of diagnostics and biochemistry. We specialize in immunofluorescence, ELISA and membrane technologies for a variety of unique and routine autoimmune and infectious diseases. Scimedx combines product development, manufacturing and distribution with a keen awareness of laboratories needs.